
It was a recognized challenge of lack of ventilators needed to face COVID-19 worldwide. Although ventilators are sparse, self-inflating manual resuscitators are widely available in-hospital services, providing a rapid response to respiratory depression. Based on this, a device (PNEUMA) [1] was designed to be a temporary solution for emergency use, allowing positive pressure ventilation through a standard self-inflating manual resuscitator, without the need for healthcare personal manually operating the resuscitator. In the first stage, the device underwent functionality and performance testing, using a calibrated lung tester. In the second stage, the usability of the device was assessed, using a clinical simulation environment, an effective method to test usability [2].
This work describes the use of a simulation environment to test the usability of a novel device to automate self-inflating manual resuscitators.
The usability study was divided into two parts: (1) participants followed a protocol with instructions for assembling and using the system in a non-clinical context (Figure 1, left panel) and (2) participants used the system in an immersive simulation environment with a clinical case scenario (Figure 1, right panel). Participants received information on how to assemble/use the system through a 4-page user manual. To monitor participants’ interaction with the system, both parts were video-recorded and questionnaires on key aspects of usability were filled out.

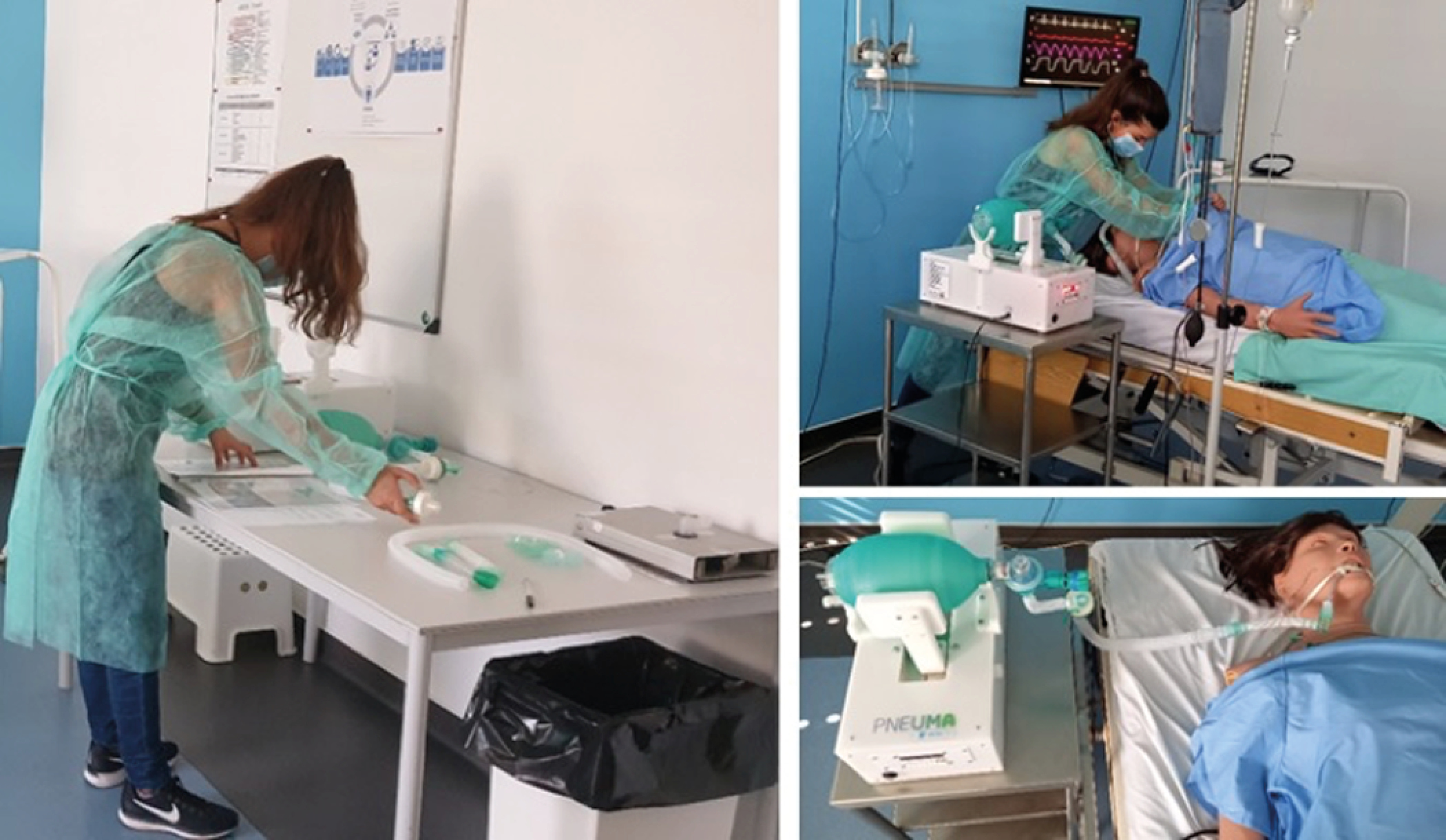
A convenience sample (two MDs and six RNs) from an intensive care unit of a tertiary Portuguese hospital participated in the test. Usability testing showed that the system was easy and timely assembled, with low complexity of use (e.g. not requiring external help). The clinical scenario tested the transition between spontaneous and mechanical ventilation, and ventilatory parameters’ control, using PNEUMA. All participants reported that the controllable parameters (I:E, RR, Vol, PIP, Plat, and PEEP) were relevant and easy to change. Participants suggested the inclusion of patient parameters such as the tidal volume and lung compliance. Participants also suggested improvements, such as the inclusion of pressure alarms and a more user-friendly interface. All participants reported that they would be willing to use the device for emergency use.
The reported study resulted in recommendations and ameliorations of the device, before its use in real settings, in the context of the COVID-19 pandemic. The use of simulation environments for device/systems’ testing provides a timely and standardized approach, enabling a safer clinical practice.
1.
2.