
In-hospital cardiac arrests that occur outside of the intensive care unit may require transportation during active cardiopulmonary resuscitation. Studies have shown that high-quality cardiopulmonary resuscitation is imperative for survival with preserved neurologic function. We sought to determine if high-quality cardiopulmonary resuscitation is maintained during simulated transportation of paediatric in-hospital cardiac arrest.
Randomized crossover simulated study of paediatric in-hospital cardiac arrest with 10 teams composed of five providers (physicians, advanced practice providers, nurses and respiratory therapists). Teams remained in a simulation room or transported the mannequin between two rooms. The primary analysis compared chest compression fraction in stationary versus transport simulations. Secondary analyses included additional cardiopulmonary resuscitation quality metrics with comparison to the 2015 American Heart Association standards.
There was no significant difference in chest compression fraction or rate between the transport and stationary groups. 92%, 72% and 26% of epochs met American Heart Association criteria for compression fraction, rate and depth, respectively. Stationary simulations were more likely to meet recommendations for combined quality metrics, including compression fraction and rate (77 vs. 53; p < 0.001) and compression fraction, rate and depth (25 vs. 7; p < 0.001).
Chest compression fraction was preserved during simulated in-hospital cardiac arrest with transport. However, the transport simulation was less likely to meet American Heart Association recommendations for combined metrics. Similar to previous cardiopulmonary resuscitation quality studies, both teams failed to meet depth requirements in the majority of simulations.
What this study adds
• This study is the first to look at American Heart Association recommendations for combined cardiopulmonary quality metrics (chest compression fraction, rate and depth) during transport of a simulated paediatric in-hospital cardiac arrest.
• Similar to previously published literature, our study found that chest compression fraction and chest compression rate are preserved in simulated transport of paediatric in-hospital cardiac arrest; however, we did find a significant difference in chest compression depth between the stationary and transport groups.
• Our study found a significant difference in chest compression depth during the first 4 minutes prior to active transport. This particular time period is subject to multiple external variables that may influence cardiopulmonary resuscitation quality.
There are an estimated 15,200 paediatric in-hospital cardiac arrests (IHCA) in the United States annually, with survival to hospital discharge occurring in up to 58% of patients [1–6]. High-quality cardiopulmonary resuscitation (CPR) has improved survival and neurologic outcomes in paediatric patients [5,7].
IHCA that occur outside of the ICU have limited resources, including a lower staff-to-patient ratio, decreased availability of code medications, intubation supplies and crash carts, as well as limited provider experience with IHCA, all of which can negatively impact CPR quality, patient survival and neurologic outcomes [8,9]. Resources become further limited when an IHCA occurs during patient transport, which may happen transporting patients for ICU admission, from a rapid response, or prior to cannulation for extracorporeal cardiopulmonary resuscitation (E-CPR) for events that occur outside of the ICU [9].
The data on CPR quality during transport are mixed, with more recent studies showing no significant difference in CPR quality [9–15]. However, almost all of the data come from out-of-hospital adult cardiac arrests [10–14]. There have only been three studies (simulation and patient-based) evaluating CPR quality during in-hospital transport, which have also found mixed results; only one included paediatric cardiac arrests [9,16,17].
At our institution patients who would require active CPR during transport would be E-CPR candidates that have a cardiac arrest outside of the ICU and would need transport to an ICU for cannulation, E-CPR candidates arresting in the ED requiring transport to the main OR for cannulation, or patients suffering a cardiac arrest during routine in-hospital transport. Our hospital’s transport and rapid response teams include one fellow or attending, one nurse and one respiratory therapist. If a rapid response becomes a code, there will be an additional 1–2 nurses available. In these instances, the teams must decide whether to transport the patients while receiving active CPR or continue the resuscitation in a resource-limited area. Based on previously published CPR studies [9–11,14,15], we hypothesize that CPR quality metrics will be preserved in simulated transport of paediatric IHCA.
This was a randomized crossover study of simulated paediatric IHCA at Texas Children’s Hospital (TCH), Houston, TX, from March 2020 to March 2021. The study was approved by the Baylor College of Medicine institutional review board with informed written consent obtained on the day of the simulation.
Teams were composed of five members: one paediatric intensive care (PICU) fellow or attending, one PICU nurse, two compressors (comprised from the following: PICU nurses, PICU advanced practice providers (APP), respiratory therapists and PICU residents) and one respiratory therapist. We determined a team of five members would be representative of the minimum number of participants needed to run a paediatric IHCA at our institution. PICU charge nurses and educators randomly chose the participants from a convenience sample of TCH employees working on the day of the scheduled simulation. The participating respiratory therapists rotate in various units of the hospital (PICU, CICU, ER), while the nurses and APPs are primarily assigned to the PICU. Pregnant and injured participants were excluded.
The simulations were conducted using Laerdal Sim Jr™ (Laerdal, Stavanger, Norway) high-fidelity mannequins. We chose this mannequin because it has the most compliant chest wall dynamics without limitations in chest compression depth (CCD) measurement [18]. After informed consent was obtained, participants were given a 5-minute verbal orientation to simulation principles including confidentiality, the basic assumption and suspension of disbelief, as well as orientation to the mannequin. The simulation was conducted in ICU simulation rooms located within the PICU at TCH. These simulation rooms are exact replicas of our ICU patient rooms; therefore, the groups did not require specific orientation to the environment. The rooms included standardized hospital crash carts (with code medications, adjunct airway supplies and intubation equipment), a Zoll-R series defibrillator with real-time audiovisual feedback available (Zoll Medical, Chelmsford, MA, USA), oxygen tanks, a transport monitor and suction supplies.
Groups were randomly assigned to start with the stationary or transport simulation using an internet-available random sequence generator program (RANDOM.ORG, Randomness and Integrity Services Ltd). After the verbal orientation, a facilitator read the scripted scenario. The scenario was the same in both simulations, except in the transport simulation; the patient was an E-CPR candidate and had to be transported between simulation rooms on different floors for cannulation. The route was approximately 160 feet and required the teams to navigate out of the simulation room, around three corners, and down four floors via the patient elevator. The team was given a brief stabilization period and then were told when it was time to transport the patient. In the stationary simulation, the patient was NOT an E-CPR candidate and the teams completed the simulation in one simulation room that ended after 10 minutes of resuscitation (Appendix 1). Participants were told the goal of the simulation was to evaluate team dynamics during a transport of simulated IHCA; they were not told the researchers were specifically evaluating CPR quality. After the facilitator read the script, the team entered the simulation room and took over compressions performed by a second facilitator. A pragmatic approach was used for team roles with the self-identified team lead assigning participant roles, including the compressor role. There was no limitation on how many participants or how often participants could perform chest compressions. Upon completion of the first 10-minute simulation the groups were given a 20-minute rest break before completing the second 10-minute simulation. There was a 5-minute debriefing at the end of the second scenario that included a refresher of AHA CPR guidelines. All scenarios were facilitated by two simulation-trained facilitators (PICU fellow (SEB) and a PICU advanced practice provider (AKL)).
Chest compression quality metrics were recorded using a CPR feedback defibrillator (R series; Zoll Medical, Chelmsford, MA, USA) with U.S. Food and Drug Administration-cleared accelerometer-based technology via the paediatric defibrillator pads. The pads are dual sensor and placed anteriorly and posteriorly to record CCD without artefact [3,19–21]. The pads were placed prior to the start of the simulation to ensure correct positioning for accurate depth measurement. Epochs were defined as 60-second segments that began after the first participant’s chest compression. For each epoch, the CCF, median CCR (CC/min) and mean CC depth (cm) were calculated and reported by Zoll RescueNet Code Review Software (Zoll Medical). For the transport simulation, active transport was defined as the period of time the stretcher was in motion. The start and stop times were recorded by the facilitators. If the teams were actively moving at any point in the epoch, it was labelled a transport epoch.
Quality metrics were based on the 2015 AHA guidelines [22] and included median chest compression rate per minute (CCR), mean CCD (cm), CCF (%) defined as the percentage of time compressions were being performed during CPR simulation, and the number of pauses greater than 10 seconds. Recorded metrics were analysed using RescueNet Code Review software (Zoll Medical). Target parameters were based on the AHA guidelines for a 6-year-old child with a target CCR of 100–120/min, CCD of 1/3 to 1/2 of the chest diameter and CCF greater than 0.80 [21]. We chose a target CCD of at least 4.4 cm based off of previously published literature of paediatric chest diameter [3,23–25]. Each epoch was evaluated for compliance for individual as well as combined CPR quality metrics.
Participant demographics were obtained after consent, prior to starting the scenario. Demographics were obtained by a self-reported survey for all team members and included height and weight (to calculate body mass index [BMI]), exercise frequency, time in current position, and how often they performed chest compressions to evaluate if those factors had an effect on CPR quality metrics [26,27].
CCF has been shown to be an independent predictor of ROSC and, therefore, based off of EMS out-of-hospital transport data and paediatric in-hospital cardiac arrest data a priori sample-size analysis predicted that we required 10 groups to detect a 20% reduction in CCF between the stationary (expected 80%) and transport group (power = 0.8, α = 0.05, SD 20%) [3,13,28–33]. Data from the Zoll R-Series defibrillator were transmitted via Wi-Fi to RescueNet Code Review. The data included CCF, CCR, CCD and pause duration greater than 10 seconds. The datum was broken down into 60-second epochs and individually analysed into median and interquartile ranges (IQR). Pause duration was manually calculated. Demographic data were analysed using the Kruskal–Wallis test. Spearman correlation was used to evaluate a correlation between demographic data and CPR quality. Pearson coefficient was used to evaluate a correlation between compressor position and CPR quality. CPR quality data were analysed using a mixed-effects model. Individual team data were analysed with a two-way ANOVA with Sidak’s multiple comparison test. CPR quality data between the two simulations were analysed using the Wilcoxon matched-pairs signed rank test. A secondary analysis comparing our CPR quality data against the current recommended AHA targets was analysed using the Fisher’s exact test. Analyses were performed with GraphPad Prism (Version 9.1.0 for macOS, GraphPad Software, La Jolla, CA, USA). A p-value of ≤0.05 was considered statistically significant.
Between March 2020 and March 2021, 10 groups completed 20 simulations, which yielded 201 60-second epochs (100 stationary and 101 transport). Data from all 20 simulations were analysed without exclusion. There were no protocol violations, but one of the transport simulations was extended to 11 minutes due to a delay in elevator availability. There were no adverse events to any study participant.
There were a total of 50 participants; however, only 36 performed chest compressions. Of those who did not perform compressions, 9 had self-identified as the team leader and 5 as the respiratory therapist. The median years of compressor clinical experience was 2 years [IQR 2, 4.3] with 53% of participants having performed compressions within the previous 12 months. All but one participant were PALS certified. There was no correlation between CPR quality and years in position, recent performance of chest compressions or BMI (r(34) = 0.31, p = 0.39; r(34) = −0.2, p = 0.58, r(34) = 0.07, p = 0.84, respectively). Team demographic data are listed in Table 1. The median time to transport the mannequin from the 12th floor to the 9th floor was 3.1 minutes [IQR 2.6, 3.3]. The bulk of the active transport occurred between minutes 4 and 8. There was a median of 4 compressor changes during both the stationary and transport simulation (stationary IQR 3–5 compressors, transport IQR 3–4.75 compressors; p = 0.8). The longest compressor interval for each team was significantly longer in the transport simulation (stationary 2.3 minutes [IQR 2.1, 3.1]; transport 2.7 minutes [IQR 2.2, 3.2], p 0.003). The majority of the groups chose to kneel next to the patient while performing CPR. There were no significant differences between compressor position and CCF or CCR, but there was a significant difference between position and CCD (r(45) = −0.99, p = 0.014).

| Average years in current position, median [IQR] | 2 [2, 4.25] |
| Performed compressions in the past 12 months (n) | 19 |
| BMI, median [IQR] | 23.65 [22.78, 25.58] |
| Self-reported exercise frequency (days per week), compressors, median [IQR] |
2.5 [1, 4.5] |
BMI, body mass index; IQR, interquartile range.
When comparing all stationary minutes to all transport minutes there was no statistically significant difference in median CCF per minute between the stationary and transport groups, nor was there a statistically significant difference in median CCR per minute. There was no significant difference in pauses greater than 10 seconds between the groups. There was a significant interaction in mean CCD related to transportation and time (F(9, 77) = 2.768, p = 0.007) as seen in Figure 1. There was a statistically significant difference in CPR quality metrics between individual group performances on the stationary and transport simulation as seen in Appendix 2.

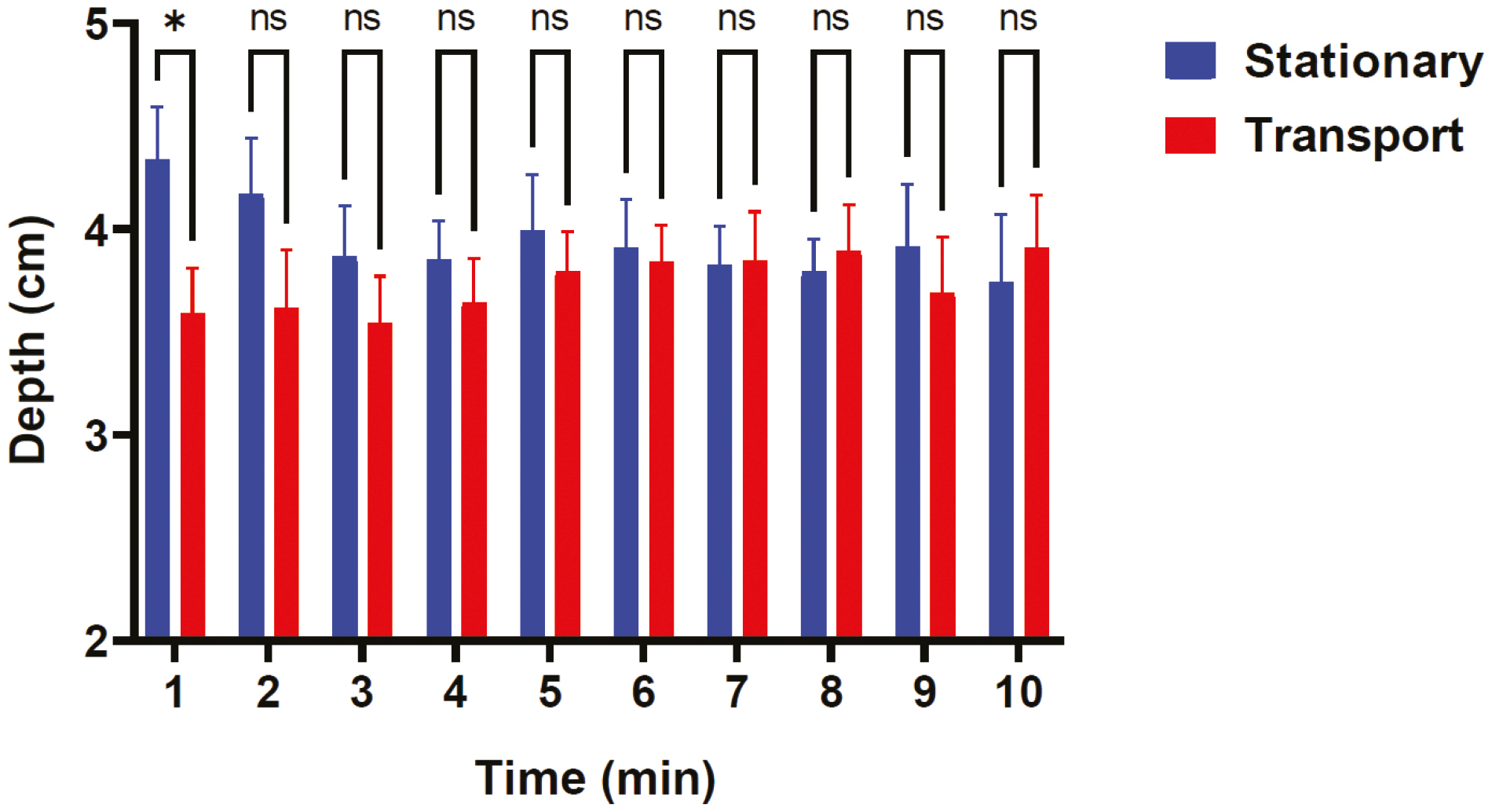
Chest compression depth over time. Mixed effects model of mean chest compression depth per minute for the 10 stationary simulations and 10 transport simulations. Predicted mean of stationary transport was 3.95 inches and the predicted mean of transport was 3.75 inches, SE of difference 0.18. Post hoc tests (using Sidak multiple comparisons) indicated that the transport group had significantly decreased mean CCD at minute 1, p = 0.028.
On analysis of all 60-second CPR metrics compared to the AHA guidelines, 92% (185/201) of epochs met AHA recommendations for CCF, 72% (144/201) met for CCR and 26% (52/201) met for CCD. 65% (130/201) of epochs met for both CCF and CCR. 16% (32/201) of epochs met for CCF, CCR and CCD. Compared to the transport simulation, the stationary simulation had a higher proportion of epochs that were compliant with AHA guidelines for rate, depth and combined AHA metrics as seen in Table 2.

| Stationary | Transport | p | |
|---|---|---|---|
| 60-second epochs (n) | 100 | 101 | |
| Epoch metrics, median [IQR] | |||
| CC fraction (%) | 95.8 [88.3, 100] | 95 [86.7, 100] | p = 0.11a |
| CC rate (CC/min) | 108.7 [103.9, 113.1] | 108.3 [100, 115.6] | p = 0.46a |
| CC depth (cm) | 3.8 [3.3, 4.5] | 3.8 [3.3, 4.2] | p = 0.02a* |
| Epoch AHA compliance, (n) | |||
| CC fraction | 96 | 89 | p = 0.06b |
| CC rate | 80 | 64 | p = 0.01b* |
| CC depth | 33 | 19 | p = 0.02b* |
| CC fraction + CC Rate | 77 | 53 | p < 0.001b* |
| CC fraction + CC rate + CC depth | 25 | 7 | p < 0.001b* |
AHA, American Heart Association; CC, chest compression; IQR, interquartile range.
aWilcoxon matched-pairs signed rank test.
bFisher’s exact test.
*For statistical significant p < 0.05.
The 60-second epochs of the transport simulation (101 epochs) were further broken down into 56 stationary epochs (pre/post transport) and 45 active transport epochs to determine whether or not there was a difference between the stationary and transport portions of the transport simulation. CPR quality data compliance was evaluated using the Fisher’s exact test. We did not find any statistical difference in any CPR quality metrics or epochs meeting AHA guidelines for individual or combined metrics (Table 3).

| Pre/post-transport | Active transport | p | |
|---|---|---|---|
| 60-second epochs (n) | 56 | 45 | |
| Epoch metrics, median [IQR] | |||
| CC fraction (%) | 95.8 [88, 100] | 92 [86.7, 100] | 0.97a |
| CC rate (CC/min) | 107.6 [100.3, 115.5] | 108.7 [97.4, 115.6] | 0.46a |
| CC depth (cm) | 3.84 [3.3, 4.2] | 3.7 [3.4, 4.1] | 0.45a |
| Epoch AHA compliance, n (%) | |||
| CC fraction | 49 (88) | 40 (89) | 0.99b |
| CC rate | 35 (63) | 29 (64) | 0.99b |
| CC depth | 9 (16) | 10 (22) | 0.45b |
| CC fraction + CC rate | 28 (50) | 24 (53) | 0.84b |
| CC fraction + CC rate + CC depth | 2 (4) | 5 (11) | 0.24b |
AHA, American Heart Association; CC, chest compression; IQR, interquartile range.
aWilcoxon matched-pairs signed rank test.
bFisher’s exact test.
This randomized crossover simulated study demonstrated that chest compression fraction and chest compression rate are maintained during simulated transport of a paediatric IHCA. There was also no difference in the number of pauses greater than 10 seconds between the stationary and transport simulations. Participants maintained adequate CPR quality during transport; therefore, it may be reasonable to transport patients undergoing active CPR for life-saving interventions.
The findings in our study are similar to previously published EMS and paediatric IHCA transport studies [9–11,14,15]. We did not find a statistically significant difference in chest compression fraction, chest compression rate, pause frequency or 60-second epochs meeting AHA recommendations for chest compression fraction on comparison of all stationary and transport epochs. The majority of epochs failed to meet the AHA recommendations for depth and combined CPR quality metrics (CCF, CCR and CCD) as seen in previous paediatric CPR quality studies [3,9].
The transport group had lower compression depth at the start of the simulation and was less likely to meet AHA metrics when compared to the stationary group. However, this difference was not present when looking at the transport and stationary epochs within the transport scenario. We believe this difference is multifactorial. It is likely this discrepancy is due to the relative infrequency of performing chest compressions during transport, provider anxiety of having to transport a patient undergoing active cardiac arrest, gathering additional medications and supplies needed to transport a patient, longer compression intervals, the absence of a dedicated CPR coach, lack of an established CPR transport simulation curriculum, compressor position and inability to minimize external stimuli due to the limited size of the resuscitation team. Although there has been significant improvement in CPR quality with implementation of real-time audiovisual feedback, the benefits may be decreased during transport of active cardiac arrests without a CPR coach [15,34–37]. In our study the Zoll defibrillator was placed at the foot of the bed. The compressors in the stationary simulation were able to directly visualize real-time feedback; however, in the transport simulation, some compressors chose to straddle the patient. This placed their backs towards the defibrillator and they lost all visual feedback. There was also more noise present in the transport simulation (moving bed, uncontrollable hallway noise, etc.) that may have negatively impacted real-time audio feedback. The addition of a CPR coach to the resuscitation team would have provided direct real-time feedback on CPR quality during the transport simulation, which may have improved CPR quality and adherence to AHA recommendations [38,39]. A recent study published by Noje et al. found that CPR quality during transport was higher in participants who have prior simulation and transport experience, and CPR quality may improve with implementation of a transport resuscitation curriculum [15]. This study achieved better CCD compared to our study further emphasizing the need for an established CPR curriculum and debriefing with CPR feedback [15]. The facilitators did not note any specific interruptions from outside ICU staff that were not participating in the simulation, but on rare occasion there was a staff member asking the team if they needed additional help with the resuscitation. While this is not uncommon in ICU resuscitations, or during transport, this may have contributed to the CPR quality difference between the stationary and transport simulation and could have been mitigated by additional team members or facilitators limiting external distractors.
Similar to the Loaec study [9], we did not find statistically significant differences in CPR quality metrics when we evaluated the pre/post transport and active transport epochs of the simulated transport IHCA. However, we did find significant differences when we looked at overall CPR quality metrics for the stationary and transport simulations. The most noticeable difference was decreased compression depth at the start of the transport simulation. During this time, the majority of teams were preparing to transport and not actively transporting the mannequin. This suggests there may be additional variables between routine IHCA and transporting an IHCA that affect CPR quality. As above, we believe these variables include anxiety regarding transport of an IHCA, determining proper CPR choreography for performing compressions during transport as well as how to switch compressors to prevent prolonged compression intervals and compressor fatigue, locating transport supplies and preparing multiple doses of code medications as these are not be readily available after you leave the room and minimizing external distractions.
Although our CPR quality data failed to meet current AHA recommendations, our data exceeded previously reported CPR quality data from pediRES-Q for children aged 1–8 years and less than 10% of epochs had pauses greater than 10 seconds [3]. However, the transport epochs failed to meet CCD and total CPR compliance and met significantly lower CPR quality metrics compared to our stationary epochs. In addition to CPR quality data, this study revealed additional areas for improvement. Our pragmatic approach allowed us to better understand how teams prepared for a cardiac arrest and to observe their CPR choreography. We found that regardless of their strategy, depth suffered in the first 4 minutes of transport. We believe this could be improved through repetitive simulation of a transported cardiac arrest, pre-planning of required medications, equipment, and transport route, as well as a dedicated CPR coach to monitor CPR quality with a focus on the preparation phase. In regards to compressor choreography, we found that the walking group had significantly lower depth than the kneeling groups. Similar results have been found in previously published manikin studies and therefore we recommend against performing compressions while walking [16,40]. CPR quality and pauses were identical in the kneeling and straddling group; however, we did find that the straddling group lost direct visualization of real-time visual feedback and had additional difficulties with compressor transitions. Interestingly, we did not find a significant difference in non-compliant pauses between the stationary and transport groups. All of the groups that had pauses during the transport simulation also had pauses during the stationary simulation; while almost all of the groups that had 0 non-compliant pauses in the transport simulation also had 0 non-compliant pauses in the stationary simulation. These groups were more likely to higher overall CPR quality, had an established compressor order and often counted before switching compressors. We feel this attention to detail is why our study differs from previously published literature [13,16]
Strengths of this study include that it utilized a pragmatic approach in regards to team participants as well as role assignments. The crossover study design randomized participants to minimize confounding variables including maturation bias that may be present on repeated simulation performances. To our knowledge, this study is the first to look at AHA recommendations for combined CPR quality metrics (CCF, CCR and CCD) during transport of a simulated IHCA.
There were several limitations to this study. This was a single-centre mannequin-based simulation study that only evaluated CPR quality metrics for a specific age group. We did not include infant or adolescent/adult mannequins. While the participants were not specifically told the study was evaluating CPR quality, the overall CPR quality metrics were higher than previously published data [3,9]. This result may have been due to the Hawthorne effect as the groups completed two nearly identical cardiac arrest scenarios with real-time audiovisual feedback available on the Zoll defibrillator. As stated above, because the majority of the compressors chose to straddle the patient, there was a discrepancy between the availability of visual CPR feedback, which may have led to a decrease in CPR quality metrics during the transport simulation. Lastly, the study was powered based on out-of-hospital EMS [13] and current paediatric IHCA [3] studies predicating a difference of 20% in CCF; however, both the transport and stationary groups exceeded the current IHCA CCF data and would have required more than 10 groups to detect a significant difference.
Larger studies are needed to determine if CPR quality during transport is comparable to overall in-hospital CPR quality, and if there are additional confounding factors that need to be accounted for, such as provider apprehension regarding transporting an active IHCA. Finally, additional studies are needed to determine if there are differences in survival to hospital discharge or neurologic outcomes in patients that received CPR in one location versus those that were transported receiving active CPR.
It may be possible to maintain high-quality CPR during transport with adequate preparation, use of real-time audiovisual feedback, implementation of a CPR coach and repeated simulation.
Supplementary data are available at The International Journal of Healthcare Simulation online.
The authors would like to thank all of the paediatric critical care fellows, advanced practice providers, residents, nurses and respiratory therapists for their participation in the simulations as well as Tom Fogarty, MD, for his help with data collection. The opinions and assertions expressed herein are those of the author(s) and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences or the Department of Defense. This work was prepared by a military or civilian employee of the U.S. Government as part of the individual’s official duties and therefore is in the public domain and does not possess copyright protection (public domain information may be freely distributed and copied; however, as a courtesy it is requested that the Uniformed Services University and the author be given an appropriate acknowledgement).
All authors conceived and designed the study. SEB conducted the simulations and collected data with AKL. SEB and FWL analysed the data. SEB drafted the manuscript. FWL, JJL and CBD contributed to critical manuscript revisions. All authors approved the final manuscript.
None declared.
The data that support the findings of this study are available within this article, figures and tables. Raw data are available from the corresponding author (SEB) upon request.
All participants have given informed written consent and the study protocol was approved by the Baylor College of Medicine institutional review board.
The authors declare no conflicts of interest.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.